The Evolution of Medical Tubing

Brevet’s advanced clear PVC and thin-wall medical tubing supports safe, efficient fluid transfer across clinical procedures. We offer custom fittings, attachments, and connectors tailored to your unique needs — from IV therapy to surgical, cosmetic, veterinary, and respiratory care.
Parallel Y Connector Provides Dual-Action Efficiency in Liposuction
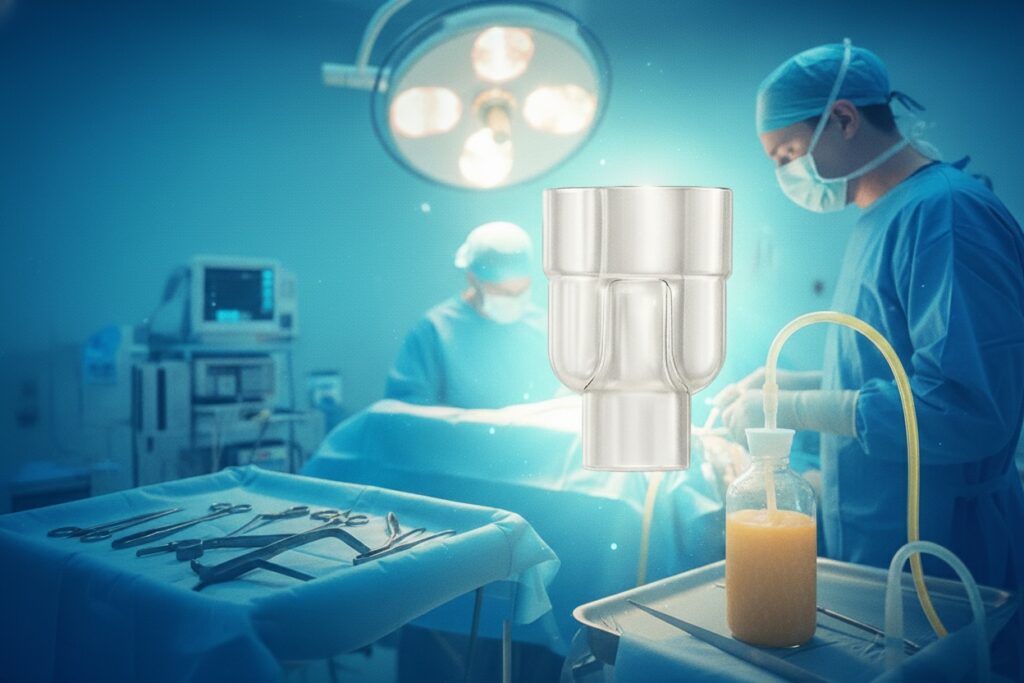
Elevate Your Liposuction Practice:
Introducing the game-changing Brevet PVC Parallel Y Connector – a meticulously cleanroom-molded, RoHS3-compliant, and fully customizable solution engineered to redefine fluid management in liposuction and plastic surgery. Experience a new era of control, designed to optimize every step of your procedure.
How PVC Connectors Keep Bioprocessing Flowing

Discover how Brevet’s gamma‑stable PVC connectors safeguard biopharma workflows — ensuring sterile, reliable fluid transfer from cell culture to final fill. Small components, big impact: protecting product integrity every step of the way.
Aseptic Assurance Importance in Fluid Component Manufacturing

When patient safety is on the line, “sterile” is the word we all want to hear. But in the high-stakes worlds of medical devices, biopharma, cosmetic formulations, and veterinary care, that word alone simply isn’t the full picture. A product can be sterilized after being exposed to all kinds of contaminants.From towering mixing vessels to delicate fill lines, lotions, serums, and creams navigate a labyrinth of tubing in all shapes and sizes. That’s where Brevet’s polycarbonate reducers step in — guiding the transition with clarity, control, and confidence.
RoHS 3 and how it applies to fluid connectors
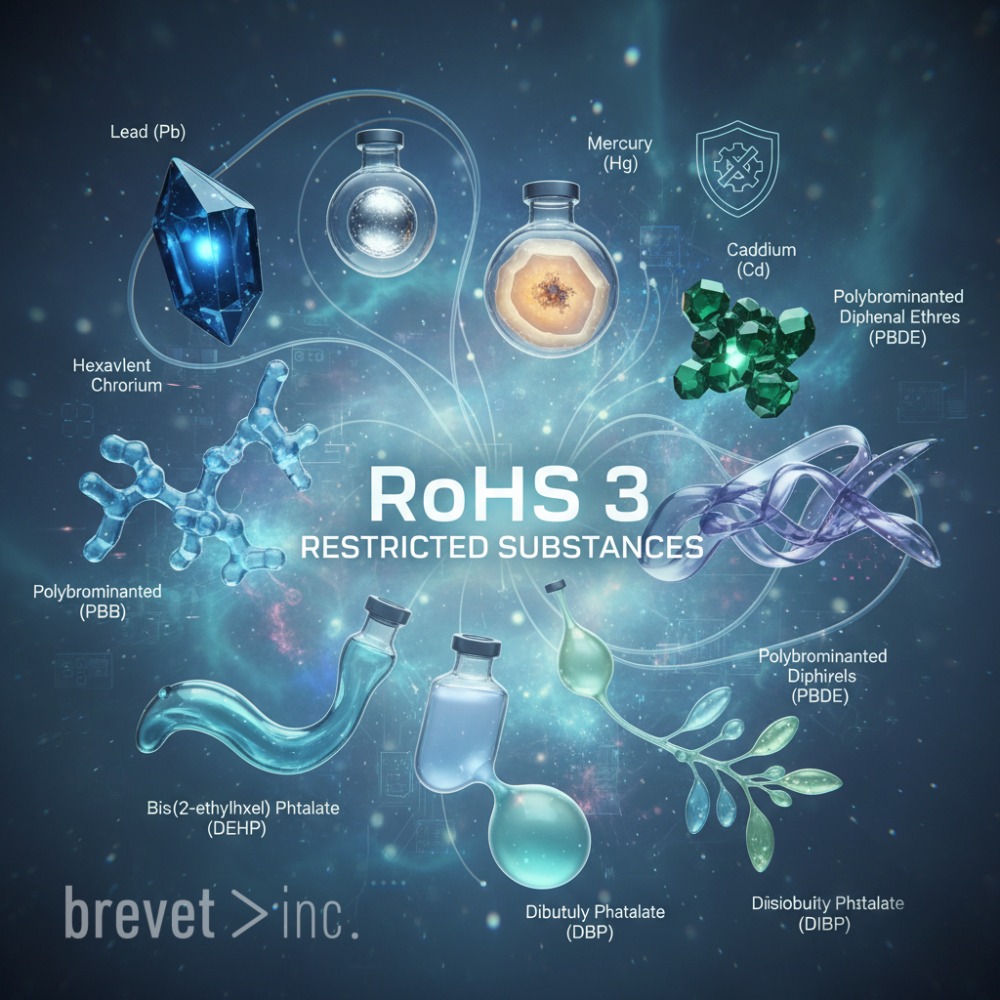
When folks hear “RoHS compliance,” they usually picture electronics — circuit boards, batteries, wires, the whole tech setup. But RoHS 3 goes way beyond that. It’s not just about what gets plugged in — it’s about what’s inside the materials we handle every day. Plastics, coatings, connectors. The stuff that flows through systems, not powers them.
From towering mixing vessels to delicate fill lines, lotions, serums, and creams navigate a labyrinth of tubing in all shapes and sizes. That’s where Brevet’s polycarbonate reducers step in — guiding the transition with clarity, control, and confidence.
How Connectors Keep Cosmetics Flowing

In cosmetics manufacturing, it’s not just about glossy packaging or velvety formulas. It’s about precision — the kind that keeps every drop moving exactly where it should. And behind that precision? Connectors.
From towering mixing vessels to delicate fill lines, lotions, serums, and creams navigate a labyrinth of tubing in all shapes and sizes. That’s where Brevet’s polycarbonate reducers step in — guiding the transition with clarity, control, and confidence.
Beyond the Split – What Makes the Parallel Y Unique

Fluid management might seem simple at first glance, connect the lines, let it flow, done. But the truth is, the shape of a connector actually plays a pretty big role in how well things move. Geometry isn’t just about aesthetics, it’s also about how fluid behaves, how the pressure balances, and also how smoothly everything actually works together. Brevet’s Parallel Y PVC Connector kind of rethinks the whole thing by offering a quieter and more balanced way to manage flow.Brevet stands at the nexus of this transformation. By providing custom, precision plastic components and single-use connectors, we empower biopharma partners to navigate this journey with the unprecedented speed and confidence that patient lives depend on.
The Future of Single-Use Sterility in Cardiac Procedures
The landscape of cardiology is undergoing a profound transformation, driven by relentless innovation and an unwavering commitment to patient safety and procedural efficiency. As cardiac interventions become increasingly sophisticated, the demand for cutting-edge solutions that support these advancements has never been greater. At the forefront of this evolution is Single-Use Technology (SUT), a paradigm shift that is redefining standards of sterility, precision, and operational excellence in cardiac care.
Brevet stands at the nexus of this transformation. By providing custom, precision plastic components and single-use connectors, we empower biopharma partners to navigate this journey with the unprecedented speed and confidence that patient lives depend on.
Accelerating Drug Development with Single-Use Fluid Paths

The work of bringing a new drug to the world is one of science’s most critical and complex missions. It requires meticulous quality control at every turn, from the lab bench to mass production. To meet this challenge, Single-Use Technology (SUT) has proven to be an absolute game-changer, accelerating development while enhancing safety.
Brevet stands at the nexus of this transformation. By providing custom, precision plastic components and single-use connectors, we empower biopharma partners to navigate this journey with the unprecedented speed and confidence that patient lives depend on.
Equine & Large Animal Single Use Technology (SUT) Benefits
As the equine market continues to demand safer, more precise, and more reliable medical solutions, Brevet is leading the way. The shift toward custom-designed, single-use fluid components is not just a passing trend—it’s a critical step forward in ensuring the best possible outcomes for our amazing large animal companions.
